Rett Rome 2018 took place from September 27-29th and gathered 170 participants from 4 continents and 17 different countries.
The aim of this very well organized scientific meeting on the 27th and 28th was to provide a platform for discussion on recent advances in basic, translational and clinical research on Rett syndrome. The meeting also intended to promote and foster collaborations.
Saturday was dedicated to parents and families and included lay summaries of the most interesting sessions of the scientific meeting.
Speakers who attracted attention the most were among others:
Adrian P. Bird, Stuart Cobb, Walter Kaufmann, Steve Kaminsky, Joost Gribnau, Jeffery Neul, Alan Percy, Alessandra Renieri and James Eubanks.
Members of the Scientific Committee responsible for the very good selection of abstracts were:
Juan Ausio, Francesco Bedogni, Angelisa Frasca, Nicoletta Landsberger and Enrico Tongiorgi.
Please find also the abstracts : Rett Rome Abstracts 2018
Stuart Cobb University of Edinburgh (UK)
An update on gene therapies for RTT
The gene therapy goal is to deliver healthy copies of the MECP2 gene with the viral vector to compensate for the mutated one in one shot.
AAV9 viral vector the same as in Avexis gene therapy cassette for RTT has already been used for some other rare neurological disorders in a clinical trial and has proven to be safe. Mecp2 protein is not a neuron specific protein but in the case of the Rett syndrome the main target is CNS (central nervous system). This is because it has been proven in the mouse model that knocking out MeCP2 on the periphery does not cause Rett like symptoms.
The most probable way of administration in clinical trials or in the future clinical practice would be intrathecal or intravenous.
How many cells do we need to target in order that the benefit of the therapy can be obvious? The answer would be as more cells than better but also we have to be very careful since two much of MECP2 can cause a MECP2 duplication syndrome with similar neurodevelopmental symptoms as Rett syndrome. The healthy copy of the gene cannot be selectively introduced only in the cells with a mutated copy of MECP2 so in the case its introduced in the cell which has a healthy copy already two much of it can be toxic. In other words gene therapy cassettes for RTT that has been developed until know have narrow therapeutic window and too much of the virally introduces MECP2 (transgene) can cause hepatotoxicity and peripheral neurotoxicity. The future developments are aiming at “de targeting” the liver.
Optimization of gene therapy cassettes can be done in different ways. One of them is the use of MINI gene which is 1/3 size of MECP2 and this makes it possible to build additional safety features in the cassette. Even with these improvements the gene therapy at the moment has suboptimal results and is not nearly as successful as a reversal experiment. As we mentioned before the reason for this is that higher doses lead to overexpression which can be toxic.
We need better vectors that can target the brain more effectively. Some are in the developmental phase with good results on mouse models and can be hopefully used for the future better gene therapy cassettes for Rett syndrome.
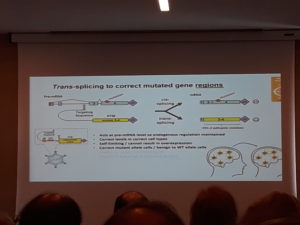
*Trans-splicing and RNA editing may in future offer superior forms of gene therapy
In trans-splicing just parts of the gene are incorporated. You can correct up until normal levels. These parts of the gene are delivered just to the cells that need it, or to those who have a mutated MECP2 copy of the gene active.
Looking further ahead RNA pinpoint gene (base) editing or
Site-directed RNA repair of endogenous Mecp2 RNA in neurons (Gel Mandel working on this) might be able to solve these problems.
Conclusions:
Concept of reversibility demonstrated for RTT (MECP2)
– profound genetic rescue
Gene therapy challenges
-delivery (targeting sufficient cells across the brain (and periphery))
-achieving appropriate transgene expression
1. avoiding ectopic expression/hotspots
2. expressing at the appropriate level for a given cell type
3. avoiding toxicity (neurotoxicity and hepatotoxicity)
4. complexities of mosaic expression
Strategies currently being tested include:
1. Novel regulated cassettes
-Feedback loops
-Enhanced endogenous regulation
2. Trans-splicing
-self limiting
-act in a cell appropriate manner
-effective across most RTT-causing mutations
Current barriers
-delivery
-adequate efficiency
Ravi Anand Newron Pharmaceutical SpA (Italy)
Sarizotan in the treatment of respiratory abnormalities in patients with RTT: new findings from an international 6-month, randomized, double-blinded placebo controlled phase III trial (STARS)
Breathing problems in RTT like apneas, hyperventilation, breath holding and air swallowing are present in most of the Rett individuals trough out their lives and are at the moment untreatable.
Apneas can lead to cyanosis, loss of consciousness, and progress to cardiorespiratory uncoupling which may lead to cardiorespiratory homeostasis/prolonged QT syndrome; sudden death, anxiety and fearfulness, gastrointestinal reflux, contribute to scoliosis, and may influence normal development of the brain in younger RTT patients.
Sudden death with no preceding symptoms has been reported in 22-26% pf deaths of RTT patients compared to 2.3% in the general population.
American Natural History Study and Australian study showed that in 15% of the cases breathing issues disappear completely upon observation with age.
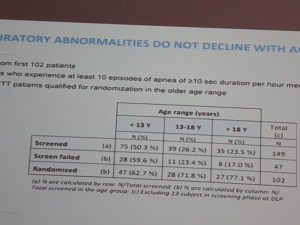
Measuring the breathing with special device, at home for long hours during the STARS clinical trial showed that breathing issues do not disappear with age over time and that the episodes and problems are more serious than we previously thought. The reason for the different results between observation of the breathing for the short time in the hospital setting and the results of the clinical trial measurements taken predominantly at home are probably due to the fact that measuring device is more accurate and that it is not enough to observe breathing for a short period of time in order to be able to see the full picture. It was also concluded that the measurements should be done at home and not in the hospital setting since RETT individuals tend to be more anxious there and this anxiety and agitation causes more hyperventilation and apneas.
Sarizotan is a selective 5-HT1A (serotonin) receptor agonist and has been tested in the phase II and III clinical trials in the past on the patients with Parkinson disease and schizophrenia and has been proven to be safe. It was tested in 1800 adult patients for 6 months and on 200 adult patients for 2 years. The given doses were 200mg/day. The max dose that was given to Rett girls in the ongoing clinical trial is 10mg/day.
Sarizotan reduced respiratory arrhythmia in pre-clinical studies in RTT female mice. Incidence of apnea and irregularity were significantly reduced and this reduction was a long term one.
In order for the Rett girls to pass recruitment criteria they should be with abnormal breathing for more than 10% of the time.
American sleep society has described how much apneas is clinically significant in adults and this is a least 10 apneas longer than 10 seconds in one hour. This is the criteria that Newron clinical trial protocol designers also used while describing recruitment criteria.
Recruitment procedure was three weeks long. Girls had to wear monitoring equipment for 6-8 hours/day 3-4 days/ week. Parents had to save the data to their computer and send it to the Data Monitoring Centre. There the data was evaluated independently. Girls who met the criteria were selected for the clinical trial after this three week trial period.
The sarizotan protocol (STARS) is a randomized, double-blind, placebo-controlled, six month study evaluating the efficacy and safety of sarizotan in RTT patients with respiratory symptoms in at least 129 patients.
Inclusion criteria:
-Females and males older than 4years, body weight more than 10 kg, meeting consensus clinical criteria confirmed by MECP mutation.
-Patients meet all of the following criteria related to breathing abnormalities:
-more than 10% of the time should be with abnormal breathing
-has at least 10 episodes of breathing dysrhythmia, defined by episodes more than 10s of breath holding (apnea) per hour during cardiorespiratory monitoring (performed with home/ambulatory monitoring system-BioRadio)
Some of the conclusions after analyzing the data are that there is no relationship between hyperventilation and apnea, contrary to some previous assumptions.
Quantitative recordings for over 18 hours in the home setting indicate that up to 70% of patients evaluated experience clinically significant apnea, minimally 10% of their time is spent without breathing.
STARS clinical trial is ongoing in the sights in US, UK, Italy, India and Australia. Anecdotal data from investigators mentions more alertness, activity and greater awareness of their surroundings for those girls who had improvements in apneas.
The official results of the trial will be published near the end of the next year.
Walter Kaufmann Emory University & Mind Institute/ University of California Davis (USA)
Clinical Trials in Rett syndrome: a critical appraisal
Dr Kaufmann has been working in the field of clinical trials for Fragile X for a long time and has also been a prime investigator for IGF1 phase I and phase II clinical trials in RTT. These trials have been completed and significant benefit has not been proven. This might have happened because the primary end points were not carefully chosen or the clinical trial population was not uniformed enough. It is also possible in his opinion that IGF1 should have been given in pulses and not continuously.
This session was a general one without talks about specific ongoing clinical trials for RTT.
We are witnessing the best times for RTT. Large numbers of potential treatments are in the pipeline. Two Phase 3 Trials are on the horizon: Trofinetide & CBD. Neuren Pharmaceuticals has joined forces with Acadia Pharmaceuticals in the development of trofinetide and plans to start Phase 3 in the second half of the next year.
New drugs: for example ANAVEX 2-73 who is Sigma 1 receptor agonist is approaching clinical phase of its development. It has demonstrated good safety, bioavailability, and tolerability in Phase 1 and Phase 2 clinical trials for other indications, but more recently preclinical studies sponsored by the Rettsyndrome.org Scout Program indicated that chronic oral daily dosing of ANAVEX 2-73 demonstrated dose-related and significant improvements in an array of behavioral and gait paradigms in the RTT mouse model.
First gene therapy clinical trial is due next year.
Despite all this great news there are still lots of issues concerning clinical trials design.
What to measure in a RTT clinical trial?
Existing outcome measures are outdated and suboptimal in his opinion. Biomarkers are not ready for prime time. Rett Syndrome Behavior Questionnaire is not good enough. It is very difficult to develop new outcome measure and this is why old ones are still used.
How to measure? Which study design is the most appropriate? FDA and EMA are more and more open for the adaptive clinical trial designs in the case of rare diseases.
Sometimes having one arm receiving a placebo is inevitable in a clinical trial and this is something parents should be prepared for. The good thing is that even if your child was in the placebo group it can be given a medication after a clinical trial is finished if you would like her to benefit from it while still in the development phase.
When to measure?
All the experts agree that therapies would have the most beneficial effect when given to the as younger girls as possible and before the regression. This however is seldom done in a clinical trial setting for the safety reasons. Young children are the most sensitive to medication and clinical trials in very young children are rare. Also regression period is not the best time to include a child into a clinical trial.
And after all we should all be aware that only a fraction of patients respond well (efficacy-side effects) to a given drug and we should keep in mind personalized approach to treatment.
Steve Kaminsky Rettsyndrome.org (USA)
IRSF’s Scout Program: an in vivo screen for potential drugs to treat RTT
Steve Kaminsky is a chief science officer at rettsyndrome.org. Pro Rett Ricerca has been funding this project from its beginning.
PsychoGenics is a Paramus, NJ-based preclinical contract research organization (CRO) and can provide access to its phenotypic discovery platforms. This includes its SmartCube system, the company’s a proprietary high throughput, high content, in vivo screening platform. This platform combines in vivo behavioral knowledge with advances in robotics, computer vision and bioinformatics to evaluate drug candidates.
https://www.rettsyndrome.org/research/our-funded-programs/scout-program?
https://www.rettsyndrome.org/file/andrea-files/Scout-Program-Update12-8-16PDF.pdf
PsychoGenics tested compounds, whether new or repurposed, through standardized preclinical studies in the heterozygous female Rett mouse model.
The issues they encountered were the cost of the mice and their slow production. The tendency was to push for oral administration of the compounds. Thing to be considered when translating the results from mouse model to humans are the difference between the permeability of the brain barrier that exists between these two species.
The goal was to fail fast the tested compounds so the tests done on the mouse are those that could be done as early as possible.
In the first tier 10-12 compounds were tested per year on 20 Rett female het (heterozygous) mice between 8-12 weeks. Tests done were BW, RotaRod, Grip strength, Startle, and Neurocube.
1-2 compounds per year passed to second tier and were tested on 20 Rett female mice. Breathing and visual response was measured.
One drug per year was tested on a tissues collection for health, survival and neurological function.
Progress to date is as follows:
– 33 compounds tested
– 5 compounds positive in Tier 1
– 2 compounds positive in Tier 2, 1 compound was not tested in Tier 2 because the company had sufficient data in these tests to move forward with the FDA, and 2 additional compounds pending Tier 2 testing
– 3 companies moved forward, filed for orphan drug status and filed for and IND for RTT at the FDA
– 1 company is in a Phase 2/3 clinical trial investigating the drug
– 2 companies planning Phase ½ clinical trials with the FDA or EMA
To date three compounds have moved through the Scout Program to the clinic. They are: 1) Newron’s Sarizotan which is actively in a clinical trial around the globe. 2) Anavex’s compound anavex 2-73 that will start in clinical trials in the USA within the next 2 months and 3) AMO’s tianeptine which has just been reviewed in Europe and is planned to be in a clinical trial there in the next year.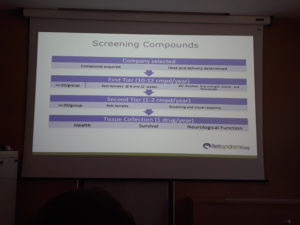
Daniele Vigli, Centre for Behavioral Sciences and Mental Health Instituto Superiore di Sanitá, Rome, (Italy)
The phytocannabinoid cannabidivarin (CBDV) rescues behavioural alterations and brain atrophy in Mecp2-308 hemizygous male mice
This study was supported by GW Pharmaceuticals based in UK.
https://www.gwpharm.com/
The study evaluated the potential therapeutic efficacy of CBDV, a non-psychotropic phytocannabinoid derived from Cannabis sativa.
The mice chosen for the study have a milder phenotype. They have been given intraperitoneal injection of CBDV.
CBDV treatment in RTT mice improved general health status, social behavior and motor skills and was also associated with normalization of reduced brain weight in RTT mice. GPR55 expression was increased in RTT mouse hippocampus.
CBDV has unknown mechanism of action but the study led to conclusion that CBDV might be a GPR55 receptor (G-protein coupled receptor 55) antagonist.
Moreover, the present study suggests GPR55 receptor as a new potential target for the treatment of RTT.
CBDV is already in clinical trials for autism and focal epilepsy and GW pharmaceuticals is planning their RTT clinical trial to start this year.
https://www.gwpharm.com/about-us/news/gw-pharmaceuticals-announces-preliminary-results-phase-2a-study-its-pipeline-compound
GW Pharmaceuticals and their product CBDV in Autism Spectrum Disorders:
10-patient investigator-initiated expanded access program for seizures associated with autism underway
Investigator-led 100 patients placebo-controlled trial in autism spectrum disorder due to commence in Q2 2018
Open label study in Rett syndrome due to commence in Q2 2018 and Phase 2 placebo-controlled trial in Rett syndrome due to commence in Q3 2018
Orphan Drug Designation from FDA for CBDV for the treatment of Rett syndrome
Alessandra Renieri Medical Genetics, University of Siena, Policlinico Le Scotte, Italy
Toward gene editing in RTT
Alessandra presented the work of her lab as a successful example of transformative gene-based medicine using CRISPR/Cas9 gene editing technology in their previously established cellular model, in fibroblasts, iPSCs, iPSC-derived neuronal precursors and neurons. Next steps will include the ongoing testing on mouse model and patient recruitment for clinical trial.
Since both decreasing as well as increasing MECP2 gene dosage is known to cause diseases in humans, “gene editing” correcting mutated allele under the native control elements appear to be the most effective and safe strategy compared to “gene replacement” which includes the addition of a normal copy of the gene under artificial control elements.
Enrico Tongiorgi Cellular and Developmental Neurobiology lab at Department of Life Sciences, University of Trieste, Italy
Repurposing Mirtazapine to target RTT
Antidepressants have emerged as potential treatments against RTT, as they could be able to normalize low monoamines transmission found in this disease. Mirtazapine is well tolerated tetracyclic antidepressant belonging to NaSSA class that specifically increases noradrenergic and serotoninergic transmission. It also has anxiolytic effect and can help with insomnia. They previously showed that this drug is able to rescue cortical atrophy, anxiety phenotype, respiratory and hearth problems in a male RTT mouse. In order to be able to move to clinical trial information on the effects of mirtazapine in female RTT mouse models and on its mechanism of action in RTT are needed. This is why mirtazapine treatment was given to 6-months-old adult female mice. Normalization of anxiety levels and a trend towards a rescue of forelimbs fine motor skills was found. No adverse events noted during this experiment. Retrospective analyses of the medical records from 11 adult RTT patients were also performed. They were previously given mirtazapine to treat anxiety and sleep disturbances. It was found that this treatment was well tolerated in patients and it induced increased motor and stereotypes scores, demonstrating a rescue in these symptoms. Also irritability and hyperactivity levels were significantly improved after mirtazapine treatment.
Using an in vitro model, involvement of the BDNF/TrkB signalling cascade in the mechanism of action of mirtazapine was found. These results strengthen the hypothesis that mirtazapine may represent and effective treatment for RTT.
Jean-Christophe Roux Aix Marseille University (France)
Calcineurin-huntingtin pathway restores BDNF trafficking and improves Mecp2 knock-out mice symptoms
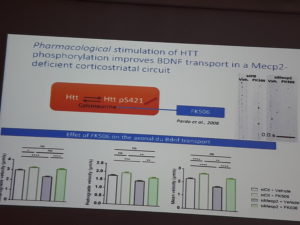
In RTT BDNF levels are lower than normal. Axonal transport of the BDNF- containing vesicles is also affected.
Huntingtin (HTT) and HTT-associated protein 1 serve as scaffolds for molecular motor complexes, transport BDNF-containing vesicles and are abnormally expressed in Mecp2 knock-out brains. In this study genetic and pharmacological (FK506 or tacrolimus, immunosuppressive drug) approaches were used to selectively stimulate HTT-dependent endogenous axonal BDNF transport in cortical neurons. Genetic and pharmacological phosphorylation improved locomotor activity, sensory-motor coordination, respiratory deficits and increased the survival of the Mecp2 mutant mice.
James Eubanks Division of Genetics and Development Krembil Research Institute Toronto, Canada
The surveillance receptor TRPM2: a novel therapeutic target for RTT?
Previous studies have revealed that cells lacking MeCP2 functional protein display elevated metabolic and oxidative stress as well as signs of altered mitochondrial function. Such alterations can cause the activation of normally inactive surveillance receptor system, such as the TRPM2 (transient receptor potential ion channel) whose activities tend to promote energy conservation by negatively regulating mTORC1. Hypoactive mTORC1 activity has been observed in several RTT model systems. mTORC1, also known as mammalian target of rapamycin complex 1 is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis.
The results of this recently finished study implicate the TRPM2 in RTT pathogenesis, and provide evidence that it could be targeted for therapeutic benefit. At the moment three compounds are known to block this channel, namely one pharmacological blocker flufenamic acid (FFA) and antifungal imidazoles clotrimazole and econazole inhibit TRPM2-mediated current.